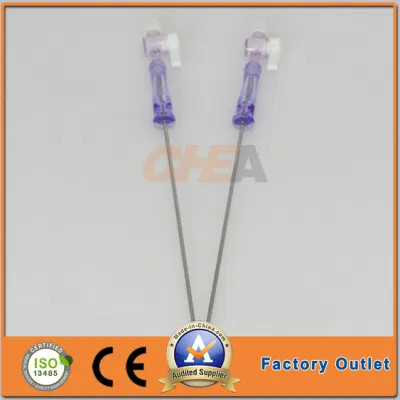
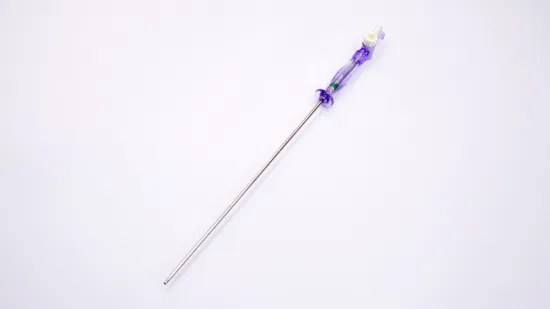
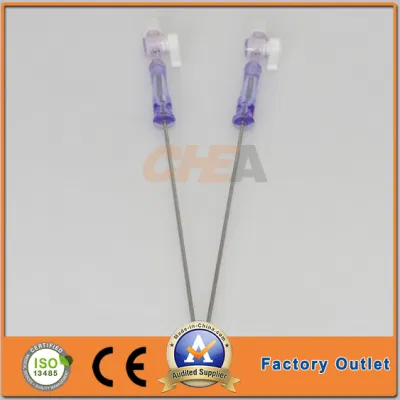
510K Cleared Disposable Sterile Trocars 3mm and 5mm Trocars for Pediatric Surgery
Description
Basic Info.
| Model NO. | T05-55-CT-S |
| OEM | Yes |
| MOQ | 1 |
| Color | Blue |
| Transport Package | Peel Pouch Packaging |
| Specification | 5mm*55mm |
| Trademark | TK |
| Origin | China |
| HS Code | 9018909000 |
| Production Capacity | 100000 |
Product Description
510K cleared disposable sterile trocars 5mm, 10mm, 12mm, 15mm trocars for abese patients,100% no air leakage, compatible to all kinds of surgical instruments.
We have specialized in medical devices and laparoscopic instruments field for over 17 years. Our products have been sold to more than 1000 top-rank hospitals in China. Our annual sales volume for trocars and retriever bags exceeds 100,000 units.
We export trocars, retriever bags and veress needles to 40 countries, most of which are European and American countries, such as the USA, UK, France, Germany, Italy, Belgium, Spain, Japan, South Korea.
We offer full range and high-quality products--We were on-site audited by FDA from 1/3/2015 to 1/13/2015, and from 7/5/2021 to 16/5/2021. We passed FDA audit and have established stable contact relationship with FDA auditors.
We have FDA Registration, CE Certificate, ISO13485:2016 Certificate, Free Sale Certificate, and Declaration of Conformity for all our products including retriever bags, trocars, veress needles.


| G T.K trocars are with special features as follows: | |
| Visible: | An endoscope can be inserted into an optical trocar to make the whole surgery process |
| Universality: | No need to have an adapter between 5mm and l5mm instruments. It is compatible to all kinds of surgical instruments. |
| Safety: | The ergonomic design of the trocar thread not only makes instruments stable but also reduces harm to skin. |
| Leak-proofness: | the universal seal can be replaced without removing the trocar cannula, ensuring 100% leak-proofness. |
| Design: | Pattern and color design is pleasing and comfortable to eyes. |
| Detachability: | The sealing components can be detached to remove large tissue or organ. |

Product Parameters
| Trocars (CT-F) | |||
| Specification | |||
| Product Code | Number of Cannula | Diameter (mm) | Working Length (mm) |
| T03-55-CT-S | 1 pc 3mm | 3 | 55 |
| T05-55-CT-S | 1 pc 5mm | 5 | 55 |
| T05-100-CT-S | 1 pc 5mm | 5 | 100 |
| T05-75-CT-F | 1 pc 5mm | 5 | 75 |
| T12-75-CT-F | 1 pc 12mm | 12 | 75 |
| T05-100-CT-F | 1 pc 5mm | 5 | 100 |
| T10-100-CT-F | 1 pc 10mm | 10 | 100 |
| T12-100-CT-F | 1 pc 12mm | 12 | 100 |
| T05-150-CT-F | 1 pc 5mm | 5 | 150 |
| T10-150-CT-F | 1 pc 10mm | 10 | 150 |
| T12-150-CT-F | 1 pc 12mm | 12 | 150 |
| 2T052-CT-F | 2 pcs 5mm | 5 | 100 |
| 2T102-CT-F | 2 pcs 10mm | 10 | 100 |
| 2T122-CT-F | 2 pcs 12mm | 12 | 100 |
| 3T52101-CT-F | 2 pcs 5mm & 1 pc 10mm | 5 & 10 | 100 |
| 4T52102-CT-F | 2 pcs 5mm & 2 pcs 10mm | 5 & 10 | 100 |
| 4T52122-CT-F | 2 pcs 5mm & 2 pcs 12mm | 5 & 12 | 100 |
| Q: Are you a factory or trading company? |
| A: Yes. We are a factory in China with 17 years surgical devices developing and manufacturing experience. |
| Q: What is the core strength of GTK Medical? |
| A: Our core strength is strong R&D Capability (more than 600 patents applied) and world first class quality (FDA 510K, CE, ISO13485:2016) management on products. |
| Q: What makes GTK Trocars stand out? |
| A: Excellent sealing performance plus excellent smooth insertion and removal of surgical instruments. |
| Q: Do you provide free samples of GTK Trocars for clinical trial use? |
| A: Yes. We are proud and confident to provide free samples for real clinical testing and evaluation. |
| Q: Do you have exported experiences to large medical devices company/companies? |
| A: Yes. We exported to more than 40 countries, most of which are based in the America and Europe. |
| Q: Do you accept factory audit prior to formal partnership? |
| A: Yes. We are proud and confident to accept factory audit. We passed US FDA on site aduits on 2015 and 2021. |
Please do not hesitate to contact me right now!Let's bring win-win business to each other~~~
Our Contact